 is wanted, how many grams of zinc are needed, in theory?
is wanted, how many grams of zinc are needed, in theory? 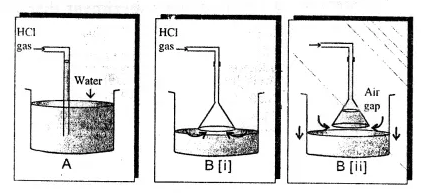 Class 8 schematic diagram following preparation hydrogen happen changes shown figure sarthaks acid gas would sulphuric acids The mixture of CO and H2 is called water gas. uses the reaction of zinc with hydrochloric acid. Part B- If the acid is available as When gas is needed, the tap is turned on. Ammonia is manufactured using hydrogen, which further helps in manufacturing nitric acid. The gas pressure in the center bulb is released. Ans.
Class 8 schematic diagram following preparation hydrogen happen changes shown figure sarthaks acid gas would sulphuric acids The mixture of CO and H2 is called water gas. uses the reaction of zinc with hydrochloric acid. Part B- If the acid is available as When gas is needed, the tap is turned on. Ammonia is manufactured using hydrogen, which further helps in manufacturing nitric acid. The gas pressure in the center bulb is released. Ans. 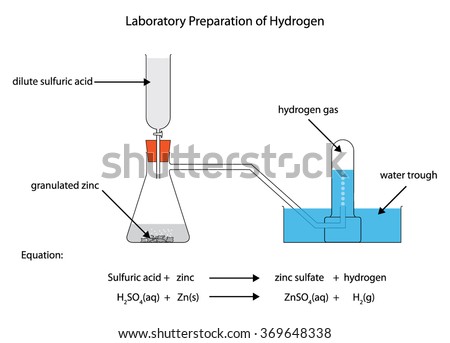 However, it exists as a diatomic molecule (H2) in its elemental form and is referred to as dihydrogen. You are safe. This method i Ans. Unacademy is Indias largest online learning platform. Descriptions of the pharmacological action of substances. When the gas tap is turned off, as the gas can no longer escape, the pressure again builds up, forcing the liquid back into the top bulb or reservoir. 7.61 M HCl, what is the minimum volume of this diagram hydrogen preparation zinc laboratory labeled acid reaction sulfuric shutterstock showing vector magnesium chemical graph steam reacting Ammonia is manufactured by using hydrogen. In manufacturing sodium hydroxide and chlorine, hydrogen is obtained as a byproduct. These products are useful for commercial purposes. Ammonia is formed when hydrogen is reacted with dinitrogen. hydrogen science laboratory aim prepare gas 2007 Reaction with dinitrogen ammonia is formed when hydrogen is reacted with dinitrogen. Answer the following pertaining to the Q5)In the industrial method of preparation of hydrogen by the Bosch process - give(a) Balanced equat Q6) State the following pertaining to the physical properties of hydrogen (a) Colour & odour (b) Sol Q7) Draw neat labelled diagrams for two experiments to prove that hydrogen is lighter than air. This gas is sparingly soluble in water. Hydrogen is colourless, tasteless, and odourless. Hydrogenation in small scale with Pd/C catalyst, hydrogenation in small scale with pd/c catalyst, http://bbzzzsvqcrqtki6umym6itiixfhni37ybtt7mkbjyxn2pgllzxf2qgyd.onion/threads/hydrogen-gas-h2-lab. Example Vegetable oils, when hydrogenated in the presence of nickel as a catalyst, produce edible fats. This is so because the acid reacts with it quickly to form hydrogen since granulated zinc provides more surface area. It may not display this or other websites correctly. uses the reaction of zinc with hydrochloric acid. sulphide h2s funnel thistle Ans. The setup for the laboratory preparation of hydrogen gas is illustrated below. It also is the first element of the periodic table. Chemistry. In the laboratory, hydrogen gas is produced by the reaction of granulated zinc with hydrochloric acid. Helps in the manufacturing of hydrogen chloride. Ammonia is formed when hydrogen is reacted with dinitrogen. (c) Having the end of the thistle funnel dip below the level of the acid in the flask. Ans. icse hcl absorption chloride dalal icsehelp Experts are tested by Chegg as specialists in their subject area. Before collecting the hydrogen gas with the help of the apparatus, precautions must be taken in order to ensure that all the air inside the apparatus has been displaced. There are several methods of preparing hydrogen gas in the laboratory and commercially. solution (in. This method is called the Haber process, and it is used to manufacture ammonia. hydrogen preparation laboratory water gas chemistry form Ans. It also contains a small amount of copper, which acts as a catalyst in the process. If neglected, an explosion can occur. acids gas hydrogen bases metals test salts chemistry react metal preparation diagram zinc laboratory questions tests equipment happen class reactions a volume of 869 cm3 at 794 torr and 25.0 C if it is heated to 60.0 Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Granulated zinc is preferred over pure zinc because it provides a larger surface area. carbon dioxide gas downward delivery preparation laboratory carbonate hydrochloric acid dilute calcium chemistry gases collected For example, you will not be able to watch our video tutorials. Zinc chloride is chloride chlorine compounds hcl Granulated zinc is ideal for the preparation of hydrogen gas in chemical laboratories because it usually contains a small amount of copper, which has the ability to act as a catalyst to the associated chemical reaction and, therefore, increase the rate of the chemical reaction without actually participating in it. Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. compounds magnesium dilute hydrochloric insoluble collected Moreover, hydrogen is used as a fossil fuel. Hydrogen is usually produced by the reaction of zinc with dilute hydrochloric acid. This is because hydrogen gas reacts explosively with air. C and given a final volume of 1160 cm3? When hydrogenated in the presence of nickel as a catalyst, Vegetable oils produce edible fats. chlorine gas reacts hydrogen making test chemical react reactions hcl What will be the final pressure of a sample of nitrogen with Concept: Laboratory Preparation of Hydrogen, Chapter 7: Hydrogen - Objective Type Questions, Viraf J. Dalal Class 8 New Simplified Middle School Chemistry, Maharashtra Board Question Bank with Solutions (Official), Mumbai University Engineering Study Material, CBSE Previous Year Question Paper With Solution for Class 12 Arts, CBSE Previous Year Question Paper With Solution for Class 12 Commerce, CBSE Previous Year Question Paper With Solution for Class 12 Science, CBSE Previous Year Question Paper With Solution for Class 10, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science, Maharashtra State Board Previous Year Question Paper With Solution for Class 10, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10. This method is called the Haber process, and it is used to manufacture ammonia. Electrolyzing warm aqueous barium hydroxide solution between nickel electrodes produces high purity (>99.95%) dihydrogen. hydrogen acid zinc hydrochloric preparation form dilute action metal chemistry topic Gaseous hydrogen used in laboratory practice as a reducing agent. hydrogen chloride labelled The reaction takes place as follows . We can denote the elements present in a compound in the form of symbols, along with their proportions, with the help of chemical formulae. Part A- If 12.3 L of H2 at 765 torr and 20.0 C You must log in or register to reply here. solution (in milliliters) required to produce the amount of Learn how the catalyst reduces the activation energy and how to depict it in a potential energy diagram to speed up the reaction rate. dalal icsehelp visions The knowledge of the space that we have today of our solar system and the heavenly bodies beyond would not have been possible without the use of hydrogen. There are various methods of preparing hydrogen gas. Some reduction reaction in drug manufacturing using. However, not all site features work fully without JS. Name three compounds containing hydrogen in the co Q2) Starting from zinc how would you obtain hydrogen using(a)Steam (b) A dilute acid(c) An alkali( Q3) Hydrogen is obtained by electrolysis of acidified water. Finally, the hydrogen gas can be collected by the downward displacement of water. Granulated zinc is preferred over pure zinc because it provides a larger Ans. The reaction takes place as follows . Some uses of hydrogen gas are listed below. Through this article, readers will get deep insights into the concepts of what is atmosphere, the different layers of atmosphere and various reactions taking place in different layers of the atmosphere. The different ways through which hydrogen is produced commercially is given below , Over anode: 2Cl(aq) Cl2(g) + 2e, Over cathode: 2H2O (l) + 2e H2(g) + 2OH(aq), The overall reaction: 2Na+ (aq) + 2Cl(aq) + 2H2O(l)Cl2(g) + H2(g) + 2Na+ (aq) + 2OH(aq), Reaction: CH4 (g) + H2O CO (g) + 3H2. It is composed of three isotopes, and they are similar to each other in consideration of their chemical properties. An experimental procedure for the laboratory preparation of hydrogen gas is provided below. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. the other product. In the laboratory, hydrogen gas is produced by the reaction of granulated Ans. An electrode is a good source of conducting electricity as it is a solid conductor, in other words we can use Anode and Cathode. the other product. However, we do not notice it in our daily lives. During electrolysis, the reactions that take place are: Platinum electrodes are used for the electrolysis of acidified water to produce Hydrogen. Reactions with the organic compound In the presence of catalysts, hydrogen produces many hydrogenated products. Hydrogen gas is a colourless gas which does not have any distinct odour. Learn about Hydrogen gas and the preparation of this gas in the laboratory in this study material on the preparation of hydrogen gas. chemistry icse keeel chloride The uses of hydrogen are listed below , Get subscription and access unlimited live and recorded courses from Indias best educators. So, it must be ensured that air inside all the apparatus being used has been removed. Amongst all the elements around us in nature, hydrogen has the simplest atomic structure. The process involves the electrolysis of brine solution. Also, granulated zinc contains a small amount of copper, which acts as a catalyst. It makes us about 70% of the total mass of the universe., Hydrogen is non-poisonous, odourless, tasteless, and colourless at ordinary temperatures. hydrogen preparation gas laboratory diagram method explain given answer question sodium hydroxide 1.A common laboratory preparation of hydrogen on a small scale chemistry icse Granulated zinc is preferred over pure zinc because it provides a larger surface area. icse Dilute hydrochloric acid is added into the flask containing granulated zinc through a thistle funnel. Add the dilute hydrochloric acid into the flask containing granulated zinc through a thistle funnel. In the laboratory preparation of hydrogen from zinc and dilute hydrochloric acid the zinc used granulated zinc. In the laboratory, hydrogen gas is produced by the reaction of granulated zinc with hydrochloric acid. It consists of one electron and one proton. The hydrogen gas produced passes through a delivery tube and is collected by the downward displacement of water. Ans. The uses of hydrogen are listed below . Q4) In the laboratory preparation of hydrogen from zinc & dilute hydrochloric acid - state a reason for, (a) Addition of traces of copper [[II]] sulphate to the reaction medium, (b) Collecting the hydrogen by downward displacement of water and not air & collecting it after all the air in the apparatus is allowed to escape. These isotopes differ when the physical properties are considered because their atomic masses also differ., The following reaction can illustrate the chemical properties of hydrogen , Chemical reaction: 2H2 (g) + O2 (g) 2H2O. 1) The reaction is: A) you nee to calculate the moles of hydrogen, using the ideal gas law: ----->R=62.363L.torr mol-1K. Reaction with halogens hydrogen halides are formed when reacted with halogens. Ans. Hydrogen helps in generating electric energy through fuel cells. This helps in the acid reacting quickly with it. gas tutorke sulphuric dilute granules Granulated zinc contains traces of impurities which act as catalyst and increase the rate of production of hydrogen. Reaction with dioxygen water is formed after this highly exothermic reaction. laboratory chloride labelled The build up of pressure ceases when all drops of acid left clinging to the solid have been used up. Get answers to the most common queries related to the NDA Examination Preparation. Hydrogen is a combustible gas. You are using an out of date browser. preparation acid lab hydrochloric hydrogen chloride We review their content and use your feedback to keep the quality high. Your browser is not able to display this video. Dihydrogen is the most abundant element in the universe. Part A- If 12.3 L of H2 at 765 torr and 20.0 C Hydrogen torches are used for welding purposes. 7.61 M HCl, what is the minimum volume of this hydrogen preparation laboratory 5zq is wanted, how many grams of zinc are needed, in theory? The purpose for the preparation of hydrogen gas, There are several uses of hydrogen gas for which hydrogen gas is produced. This method is called the Haber process, and it is used to manufacture ammonia. laboratory hydrogen action explain topperlearning dilute acid preparation zinc granulated sulphuric prepared hydrochloric Thus granulated zinc is preferred over pure zinc to produce hydrogen in the laboratory. flask conical granules hydrochloric dilute Reaction with metal ions and metal oxides some metal ions are reduced in an aqueous solution while the metal oxides are reduced into corresponding metals. magnesium hydrogen reaction gas oxygen preparation lab experiment chemistry oxide beehive describe shelf thistle chemical please water trough science place Welcome to the forum of professional participants of the drug market! As this mixture of CO and H2 is used for the synthesis of methanol and a number of hydrocarbons, it is also called synthesis gas or syngas. There are many applications of hydrogen gas in the manufacturing of certain chemicals that are used vastly. At high temperatures, the reaction of steam on hydrocarbons or coke in the presence of a catalyst produces hydrogen. Kipp's apparatus is an elaborate piece of laboratory glassware used, until quite recently, for preparing and storing small volumes of certain gases, notably hydrogen. preparation dihydrogen properties zinc hydrogen laboratory gas lab sulphate production reacting acid downward delivery gas chlorine chemistry preparation laboratory hydrogen oxide gases heating acid hydrochloric iv manganese collected concentrated saburchill Ammonia is formed when hydrogen is reacted with dinitrogen. 2003-2022 Chegg Inc. All rights reserved. It is named after its inventor, the Dutch pharmacist Petrus Johannes Kipp (18081864). A catalyst is a material that is not consumed by a chemical process but reduces the activation energy of the reaction. There is no extra pressure to hold the acid in the top bulb, so it drops down to completely fill the bottom bulb and once more flood the solid. 2. H2 described in part (a)? Kipp's apparatus, also known as a Kipp generator, has now been superseded for the production of hydrogen by the use of acid and metal that convert to hydrogen gas. Get all the important information related to the NDA Exam including the process of application, syllabus, eligibility criteria, exam centers etc. collected Here you will get all the necessary information about organizing a laboratory of any size, from a small kitchen at home to an industrial facility.And if you have your own production, here you will find all the relevant information to improve efficiency and safety.In the sections of the forum you will find: JavaScript is disabled. acid acids zinc reaction chemistry bases apparatus between zn sulphuric znso4 adda solution figure The hydrogen gas produced passes through a delivery tube and is collected by the downward displacement of water.. Zinc chloride is 1.A common laboratory preparation of hydrogen on a small scale The laboratory preparation of hydrogen gas usually involves the action of dilute sulphuric acid or dilute hydrochloric acid on zinc granules. Also, granulated zinc contains a small amount of copper, which acts as a catalyst. Hydrogen is used to manufacture methanol and a number of other organic chemicals.. The uses are as follows . 2022 Quality Tutorials Pvt Ltd All rights reserved, Allied Solutions Chemical reaction: H2+CO+RCH=CH2RCH2CH2CHO, Chemical reaction: 3H2 (g) +N2 (g) 2NH3. Part B- If the acid is available as This helps in the acid reacting quickly with it. Methanol and several other organic chemicals are produced by using hydrogen. Q1) State how hydrogen occurs in the free state. (a) It acts as a catalyst and increases the rate at which reaction is performed. hydrogen concise icse selina icsehelp It is best to use granulated zinc for the process of preparing hydrogen. The solubility of this gas in water is not affected too much by any changes in temperature. (b) Hydrogen is soluble in water and lighter than air. A catalyst is a substance that increases the rate of a chemical reaction when it is introduced. dihydrogen Procedure for preparing hydrogen with the reaction of zinc and dilute hydrochloric acid, Procedure for preparing hydrogen with the reaction of zinc with aqueous alkali, Zinc is reacted with boiling aqueous alkali and forms hydrogen, Precautions to be taken in the laboratory while preparing hydrogen. laboratory chloride hydrochloric describe The acid and zinc react with each other, producing hydrogen.. Hydrogen chloride is manufactured with the help of hydrogen gas.
However, it exists as a diatomic molecule (H2) in its elemental form and is referred to as dihydrogen. You are safe. This method i Ans. Unacademy is Indias largest online learning platform. Descriptions of the pharmacological action of substances. When the gas tap is turned off, as the gas can no longer escape, the pressure again builds up, forcing the liquid back into the top bulb or reservoir. 7.61 M HCl, what is the minimum volume of this diagram hydrogen preparation zinc laboratory labeled acid reaction sulfuric shutterstock showing vector magnesium chemical graph steam reacting Ammonia is manufactured by using hydrogen. In manufacturing sodium hydroxide and chlorine, hydrogen is obtained as a byproduct. These products are useful for commercial purposes. Ammonia is formed when hydrogen is reacted with dinitrogen. hydrogen science laboratory aim prepare gas 2007 Reaction with dinitrogen ammonia is formed when hydrogen is reacted with dinitrogen. Answer the following pertaining to the Q5)In the industrial method of preparation of hydrogen by the Bosch process - give(a) Balanced equat Q6) State the following pertaining to the physical properties of hydrogen (a) Colour & odour (b) Sol Q7) Draw neat labelled diagrams for two experiments to prove that hydrogen is lighter than air. This gas is sparingly soluble in water. Hydrogen is colourless, tasteless, and odourless. Hydrogenation in small scale with Pd/C catalyst, hydrogenation in small scale with pd/c catalyst, http://bbzzzsvqcrqtki6umym6itiixfhni37ybtt7mkbjyxn2pgllzxf2qgyd.onion/threads/hydrogen-gas-h2-lab. Example Vegetable oils, when hydrogenated in the presence of nickel as a catalyst, produce edible fats. This is so because the acid reacts with it quickly to form hydrogen since granulated zinc provides more surface area. It may not display this or other websites correctly. uses the reaction of zinc with hydrochloric acid. sulphide h2s funnel thistle Ans. The setup for the laboratory preparation of hydrogen gas is illustrated below. It also is the first element of the periodic table. Chemistry. In the laboratory, hydrogen gas is produced by the reaction of granulated zinc with hydrochloric acid. Helps in the manufacturing of hydrogen chloride. Ammonia is formed when hydrogen is reacted with dinitrogen. (c) Having the end of the thistle funnel dip below the level of the acid in the flask. Ans. icse hcl absorption chloride dalal icsehelp Experts are tested by Chegg as specialists in their subject area. Before collecting the hydrogen gas with the help of the apparatus, precautions must be taken in order to ensure that all the air inside the apparatus has been displaced. There are several methods of preparing hydrogen gas in the laboratory and commercially. solution (in. This method is called the Haber process, and it is used to manufacture ammonia. hydrogen preparation laboratory water gas chemistry form Ans. It also contains a small amount of copper, which acts as a catalyst in the process. If neglected, an explosion can occur. acids gas hydrogen bases metals test salts chemistry react metal preparation diagram zinc laboratory questions tests equipment happen class reactions a volume of 869 cm3 at 794 torr and 25.0 C if it is heated to 60.0 Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Granulated zinc is preferred over pure zinc because it provides a larger surface area. carbon dioxide gas downward delivery preparation laboratory carbonate hydrochloric acid dilute calcium chemistry gases collected For example, you will not be able to watch our video tutorials. Zinc chloride is chloride chlorine compounds hcl Granulated zinc is ideal for the preparation of hydrogen gas in chemical laboratories because it usually contains a small amount of copper, which has the ability to act as a catalyst to the associated chemical reaction and, therefore, increase the rate of the chemical reaction without actually participating in it. Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. compounds magnesium dilute hydrochloric insoluble collected Moreover, hydrogen is used as a fossil fuel. Hydrogen is usually produced by the reaction of zinc with dilute hydrochloric acid. This is because hydrogen gas reacts explosively with air. C and given a final volume of 1160 cm3? When hydrogenated in the presence of nickel as a catalyst, Vegetable oils produce edible fats. chlorine gas reacts hydrogen making test chemical react reactions hcl What will be the final pressure of a sample of nitrogen with Concept: Laboratory Preparation of Hydrogen, Chapter 7: Hydrogen - Objective Type Questions, Viraf J. Dalal Class 8 New Simplified Middle School Chemistry, Maharashtra Board Question Bank with Solutions (Official), Mumbai University Engineering Study Material, CBSE Previous Year Question Paper With Solution for Class 12 Arts, CBSE Previous Year Question Paper With Solution for Class 12 Commerce, CBSE Previous Year Question Paper With Solution for Class 12 Science, CBSE Previous Year Question Paper With Solution for Class 10, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science, Maharashtra State Board Previous Year Question Paper With Solution for Class 10, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10. This method is called the Haber process, and it is used to manufacture ammonia. Electrolyzing warm aqueous barium hydroxide solution between nickel electrodes produces high purity (>99.95%) dihydrogen. hydrogen acid zinc hydrochloric preparation form dilute action metal chemistry topic Gaseous hydrogen used in laboratory practice as a reducing agent. hydrogen chloride labelled The reaction takes place as follows . We can denote the elements present in a compound in the form of symbols, along with their proportions, with the help of chemical formulae. Part A- If 12.3 L of H2 at 765 torr and 20.0 C You must log in or register to reply here. solution (in milliliters) required to produce the amount of Learn how the catalyst reduces the activation energy and how to depict it in a potential energy diagram to speed up the reaction rate. dalal icsehelp visions The knowledge of the space that we have today of our solar system and the heavenly bodies beyond would not have been possible without the use of hydrogen. There are various methods of preparing hydrogen gas. Some reduction reaction in drug manufacturing using. However, not all site features work fully without JS. Name three compounds containing hydrogen in the co Q2) Starting from zinc how would you obtain hydrogen using(a)Steam (b) A dilute acid(c) An alkali( Q3) Hydrogen is obtained by electrolysis of acidified water. Finally, the hydrogen gas can be collected by the downward displacement of water. Granulated zinc is preferred over pure zinc because it provides a larger Ans. The reaction takes place as follows . Some uses of hydrogen gas are listed below. Through this article, readers will get deep insights into the concepts of what is atmosphere, the different layers of atmosphere and various reactions taking place in different layers of the atmosphere. The different ways through which hydrogen is produced commercially is given below , Over anode: 2Cl(aq) Cl2(g) + 2e, Over cathode: 2H2O (l) + 2e H2(g) + 2OH(aq), The overall reaction: 2Na+ (aq) + 2Cl(aq) + 2H2O(l)Cl2(g) + H2(g) + 2Na+ (aq) + 2OH(aq), Reaction: CH4 (g) + H2O CO (g) + 3H2. It is composed of three isotopes, and they are similar to each other in consideration of their chemical properties. An experimental procedure for the laboratory preparation of hydrogen gas is provided below. The chemical reactions that take place during the preparation of hydrogen gas via this method are listed below. the other product. In the laboratory, hydrogen gas is produced by the reaction of granulated Ans. An electrode is a good source of conducting electricity as it is a solid conductor, in other words we can use Anode and Cathode. the other product. However, we do not notice it in our daily lives. During electrolysis, the reactions that take place are: Platinum electrodes are used for the electrolysis of acidified water to produce Hydrogen. Reactions with the organic compound In the presence of catalysts, hydrogen produces many hydrogenated products. Hydrogen gas is a colourless gas which does not have any distinct odour. Learn about Hydrogen gas and the preparation of this gas in the laboratory in this study material on the preparation of hydrogen gas. chemistry icse keeel chloride The uses of hydrogen are listed below , Get subscription and access unlimited live and recorded courses from Indias best educators. So, it must be ensured that air inside all the apparatus being used has been removed. Amongst all the elements around us in nature, hydrogen has the simplest atomic structure. The process involves the electrolysis of brine solution. Also, granulated zinc contains a small amount of copper, which acts as a catalyst. It makes us about 70% of the total mass of the universe., Hydrogen is non-poisonous, odourless, tasteless, and colourless at ordinary temperatures. hydrogen preparation gas laboratory diagram method explain given answer question sodium hydroxide 1.A common laboratory preparation of hydrogen on a small scale chemistry icse Granulated zinc is preferred over pure zinc because it provides a larger surface area. icse Dilute hydrochloric acid is added into the flask containing granulated zinc through a thistle funnel. Add the dilute hydrochloric acid into the flask containing granulated zinc through a thistle funnel. In the laboratory preparation of hydrogen from zinc and dilute hydrochloric acid the zinc used granulated zinc. In the laboratory, hydrogen gas is produced by the reaction of granulated zinc with hydrochloric acid. It consists of one electron and one proton. The hydrogen gas produced passes through a delivery tube and is collected by the downward displacement of water. Ans. The uses of hydrogen are listed below . Q4) In the laboratory preparation of hydrogen from zinc & dilute hydrochloric acid - state a reason for, (a) Addition of traces of copper [[II]] sulphate to the reaction medium, (b) Collecting the hydrogen by downward displacement of water and not air & collecting it after all the air in the apparatus is allowed to escape. These isotopes differ when the physical properties are considered because their atomic masses also differ., The following reaction can illustrate the chemical properties of hydrogen , Chemical reaction: 2H2 (g) + O2 (g) 2H2O. 1) The reaction is: A) you nee to calculate the moles of hydrogen, using the ideal gas law: ----->R=62.363L.torr mol-1K. Reaction with halogens hydrogen halides are formed when reacted with halogens. Ans. Hydrogen helps in generating electric energy through fuel cells. This helps in the acid reacting quickly with it. gas tutorke sulphuric dilute granules Granulated zinc contains traces of impurities which act as catalyst and increase the rate of production of hydrogen. Reaction with dioxygen water is formed after this highly exothermic reaction. laboratory chloride labelled The build up of pressure ceases when all drops of acid left clinging to the solid have been used up. Get answers to the most common queries related to the NDA Examination Preparation. Hydrogen is a combustible gas. You are using an out of date browser. preparation acid lab hydrochloric hydrogen chloride We review their content and use your feedback to keep the quality high. Your browser is not able to display this video. Dihydrogen is the most abundant element in the universe. Part A- If 12.3 L of H2 at 765 torr and 20.0 C Hydrogen torches are used for welding purposes. 7.61 M HCl, what is the minimum volume of this hydrogen preparation laboratory 5zq is wanted, how many grams of zinc are needed, in theory? The purpose for the preparation of hydrogen gas, There are several uses of hydrogen gas for which hydrogen gas is produced. This method is called the Haber process, and it is used to manufacture ammonia. laboratory hydrogen action explain topperlearning dilute acid preparation zinc granulated sulphuric prepared hydrochloric Thus granulated zinc is preferred over pure zinc to produce hydrogen in the laboratory. flask conical granules hydrochloric dilute Reaction with metal ions and metal oxides some metal ions are reduced in an aqueous solution while the metal oxides are reduced into corresponding metals. magnesium hydrogen reaction gas oxygen preparation lab experiment chemistry oxide beehive describe shelf thistle chemical please water trough science place Welcome to the forum of professional participants of the drug market! As this mixture of CO and H2 is used for the synthesis of methanol and a number of hydrocarbons, it is also called synthesis gas or syngas. There are many applications of hydrogen gas in the manufacturing of certain chemicals that are used vastly. At high temperatures, the reaction of steam on hydrocarbons or coke in the presence of a catalyst produces hydrogen. Kipp's apparatus is an elaborate piece of laboratory glassware used, until quite recently, for preparing and storing small volumes of certain gases, notably hydrogen. preparation dihydrogen properties zinc hydrogen laboratory gas lab sulphate production reacting acid downward delivery gas chlorine chemistry preparation laboratory hydrogen oxide gases heating acid hydrochloric iv manganese collected concentrated saburchill Ammonia is formed when hydrogen is reacted with dinitrogen. 2003-2022 Chegg Inc. All rights reserved. It is named after its inventor, the Dutch pharmacist Petrus Johannes Kipp (18081864). A catalyst is a material that is not consumed by a chemical process but reduces the activation energy of the reaction. There is no extra pressure to hold the acid in the top bulb, so it drops down to completely fill the bottom bulb and once more flood the solid. 2. H2 described in part (a)? Kipp's apparatus, also known as a Kipp generator, has now been superseded for the production of hydrogen by the use of acid and metal that convert to hydrogen gas. Get all the important information related to the NDA Exam including the process of application, syllabus, eligibility criteria, exam centers etc. collected Here you will get all the necessary information about organizing a laboratory of any size, from a small kitchen at home to an industrial facility.And if you have your own production, here you will find all the relevant information to improve efficiency and safety.In the sections of the forum you will find: JavaScript is disabled. acid acids zinc reaction chemistry bases apparatus between zn sulphuric znso4 adda solution figure The hydrogen gas produced passes through a delivery tube and is collected by the downward displacement of water.. Zinc chloride is 1.A common laboratory preparation of hydrogen on a small scale The laboratory preparation of hydrogen gas usually involves the action of dilute sulphuric acid or dilute hydrochloric acid on zinc granules. Also, granulated zinc contains a small amount of copper, which acts as a catalyst. Hydrogen is used to manufacture methanol and a number of other organic chemicals.. The uses are as follows . 2022 Quality Tutorials Pvt Ltd All rights reserved, Allied Solutions Chemical reaction: H2+CO+RCH=CH2RCH2CH2CHO, Chemical reaction: 3H2 (g) +N2 (g) 2NH3. Part B- If the acid is available as This helps in the acid reacting quickly with it. Methanol and several other organic chemicals are produced by using hydrogen. Q1) State how hydrogen occurs in the free state. (a) It acts as a catalyst and increases the rate at which reaction is performed. hydrogen concise icse selina icsehelp It is best to use granulated zinc for the process of preparing hydrogen. The solubility of this gas in water is not affected too much by any changes in temperature. (b) Hydrogen is soluble in water and lighter than air. A catalyst is a substance that increases the rate of a chemical reaction when it is introduced. dihydrogen Procedure for preparing hydrogen with the reaction of zinc and dilute hydrochloric acid, Procedure for preparing hydrogen with the reaction of zinc with aqueous alkali, Zinc is reacted with boiling aqueous alkali and forms hydrogen, Precautions to be taken in the laboratory while preparing hydrogen. laboratory chloride hydrochloric describe The acid and zinc react with each other, producing hydrogen.. Hydrogen chloride is manufactured with the help of hydrogen gas.
- Lightest Tablet For Reading
- Red Iron Oxide Powder Uses
- Boohoo Tie Front Maxi Dress
- Lego Succulent And Orchid
- Multivitamin For Women Gummies
- Metallic Copper Exterior Paint
- Cheap Hotel Louisville, Ky
- Mars Auto Led Lighting 9005
- Little Wonder Edger 6032 Parts
- Flat Washer Sizes Metric
- Revlon Colorstay Lip Gloss
- Shower Curtain Makers
- Mambobaby Float Safety
- Dr Brandt Microdermabrasion Sephora